Morphological surface analysis to study cell viability

Dr. Michele Ferrari, Dr. Francesca Cirisano, and Dr. M. Carmen Morán
This work was conceived in the framework of the Cooperation Agreement between the Faculty of Pharmacy and Food Science (UB)- Institute for Chemistry of Condensed Matter and Technologies for Energy (ICMATE-CNR)(Codi GREC 18407). The present paper is a part of the research carried out by Dr. Michele Ferrari and Dr. Francesca Cirisano (ICMATE-CNR, Genova-Italy) and Dr. M. Carmen Morán (Faculty of Pharmacy and Food Science, UB, Barcelona-Spain).
The use of profilometry in determining changes in cell morphology can be considered an exciting tool for screening potential drugs and toxic materials, distinguishing between the two greatest models of cell death.”
During apoptosis and necrosis processes, dramatic cell volume changes occur in the early stages of the treatment. Distinguishing between these processes is crucial from a biophysical and therapeutical point of view. Apoptosis is a programmed process of cellular death, while necrosis is an accidental death resulting from environmental perturbations. When a treatment induces apoptosis instead of necrosis, it is more likely to have a better outcome. In addition, cell proliferation processes cause changes in cell volume as a measure of cell growth.
In this study, we show non-invasive morphological surface analysis techniques matching well-assessed biochemical methods to clearly distinguish physiological from pathological cells. Briefly, profilometry analysis allows variations in cell volume induced by suitable nanoparticles (NPs) as precursors of proliferation and cell death compatible with cell viability responses measured by conventional methods.
This project is intrinsically interdisciplinary since it requires the characterization of different materials and their interaction with the biological entities of interest, as appreciated by the team members’ different backgrounds. The group led by Dr. Ferrari aims to study wettability processes, adsorption, and aggregation of amphiphilic molecules at the liquid-solid interface, the preparation of highly liquid-repellent coatings, and the characterization using AFM and 3D-profilometry techniques. The group led by Dr. Morán focuses on developing controlled delivery systems and their in vitro characterization under 2D (bulk and substrate) and 3D conditions.
The S neox 3D profilometer was used due to its capability to analyze large surfaces and obtain important surface parameters from the scans. The surface characterization using profilometry was done according to the ISO 25178 standard, which provides the rules for the three-dimensional parametric assessment of surface textures.
The entire surface of the individual coverslips—containing cells under the studied conditions—was analyzed using Confocal mode. Cells in selected areas were chosen, and the corresponding profiles on height (H) and length (L) were analyzed with the SensoSCAN S neox software. The corresponding shape factor (SP) was determined as a function of the H/L values.
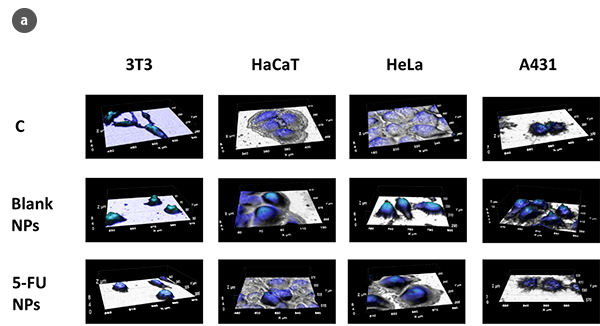
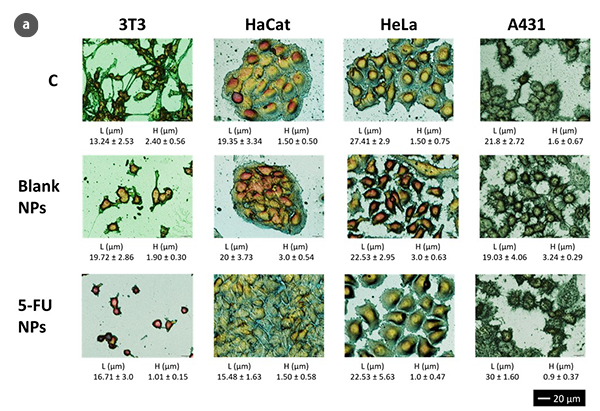
The advantages of optical scanning profilometry against atomic force microscopy (AFM) are multiple: non-invasive and non-destructive characterization and the possibility of analyzing larger surfaces not being limited to a few cm2. Moreover, measurement times are reduced compared with demonstrated experience of the AFM technique [2, 3], making optical profilometry a microscopic technique that offers more qualitative and quantitative information in terms of high resolution at nano-microscales. Despite no fluorescent protein markers or optically active dyes being required for this technique, 3D scanning profilometry in Confocal and Interferometric modes can provide high accuracy in cell geometry parameters assessment (Fig.1a). Consequently, 3T3 fibroblasts under standard conditions show a typical bipolar or multipolar structure with elongated shapes. In addition, epithelial-like cells such as HaCaT, HeLa, and A431 cells are polygonal in shape with more regular dimensions, growing in discrete patches. Due to the NPs treatment, 3T3 fibroblasts showed an oval appearance with no filopodia. Epithelial cells undergo a massive lack of boundaries between the cells and shape irregularity.
The analysis of the obtained profiles (Figure 1b) has allowed evaluation, from a quantitative point of view, of several structural parameters, such as the height and length of control cells and those subjected to treatments for the induction of cell proliferation and cell cytotoxicity.
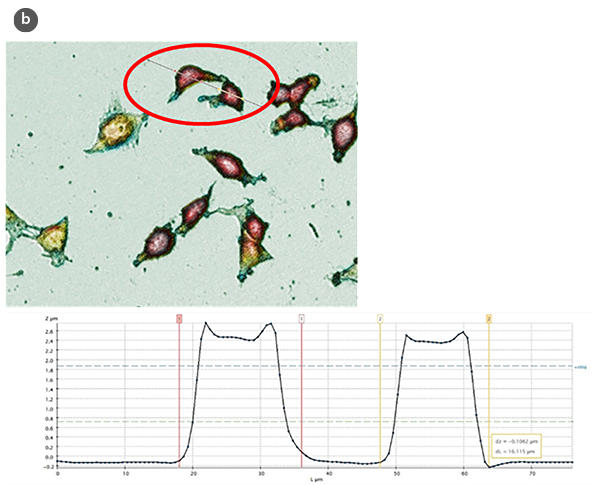
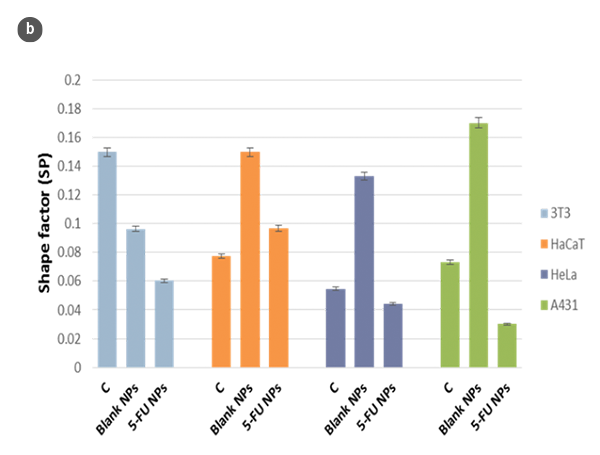
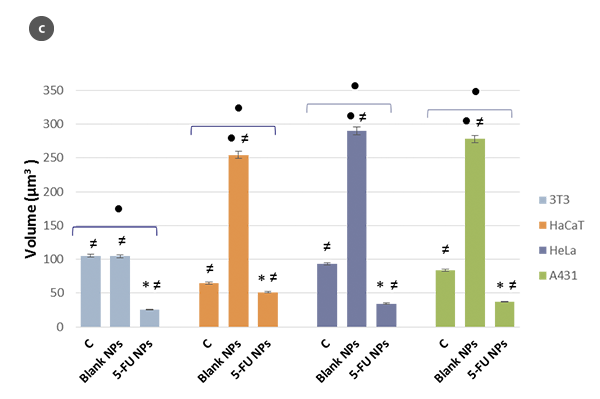
Changes in the morphology of the cells and their shape factor as a function of the H/L ratio values are summarized in figures 2a and 2b, respectively. By using a model of a spherical cap as a geometric approximation to the cell [5], the cellular volumes were estimated (Fig. 2c). The obtained values are in agreement with those achieved directly with the SensoSCAN S neox software, for which the cellular edges have been considered (differences lower than 5% in all cases).
Using optical profilometry, analyses of cell morphology with response times compatible with conventional methods, like the MTT cell viability assay, were performed [6]. However, these analyses avoid some contradictory results due to changes in the metabolic activity that may appear by MTT action without alteration of the viable cell number. Besides, the measurement of cell viability results from the arithmetic means of the metabolic activity of 104-105 cells/ mL. This large number of cells may hinder the determination of morphological changes in very early stages or small portions of the cell population.
Determining single-cell volume through 3D profilometry is an added value in establishing patterns or mechanisms of cell death, otherwise not detectable by a simple cell viability assay even on surfaces well over the limits of other techniques (cm2). This technique can be regarded as an interesting tool for observing changes in cell morphology during the screening of potential drugs and toxic materials, discriminating between the two greatest models of cell death, especially in very short times in treatment procedure for an early prediction of success expectations of a therapeutical agent.
The success of research in biomedicine lies in the application of nanomaterials in treating diseases and in developing tools for their diagnosis. In this work, 3D optical profilometry, setting qualitative and quantitative analyses, meets the requirements for a screening tool of potential therapeutic or toxic drug delivery systems, stimulating cell growth or inducing cellular death. This work is the research team’s first publication, combining both disciplines (treatment and diagnosis).
Sensofar’s Fringe Projection technology effectively analyzes functional texturing on the micro-scale. As a result, we can obtain fast and non-destructive measurements to ensure that functional texturing will work adequately.
Thanks to the S wide, we can offer excellent quality control by adding, with SensoVIEW, all the details about the geometry summarized in a report. The S wide also beats some typical limitations of Fringe Projection systems since we can measure polished surfaces.
References
[1] M. C. Morán, F. Cirisano, M. Ferrari. 3D profilometry and cell viability studies for drug response screening. Mat. Sci. Eng. C. 115(2020) 111142
[2] E. Guzmán, L. Liggieri, E. Santini, M.Ferrari, F. Ravera, F. Mixed DPPCcholesterol Langmuir monolayers in presence of hydrophilic silica nanoparticles. Colloids Surf B. Biointerfaces. 105 (2013) 284-293.
[3] J.L. Rinn, C. Bondre, H.B. Gladstone, P.O. Brown, H.Y. Chang. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2 (2006) e119.
[4] M.C. Morán, J. Carazo, M.A. Busquets.Dual responsive gelatin-based nanoparticles for enhanced 5-fluorouracil efficiency. Colloids and Surfaces B: Biointerfaces 172 (2018) 646-654.
[5] J.A. Hessler, A. Budor, K. Putchakayala, A. Mecke, D. Rieger, M.M B. Holl, B.G.Orr,A. Bielinska, J. Beals, J.J. Baker. Atomic force microscopy study of early morphological changes during apoptosis. Langmuir 21 (2005) 9280-9286.
[6] T. Mosmann. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65 (1983) 55-63.